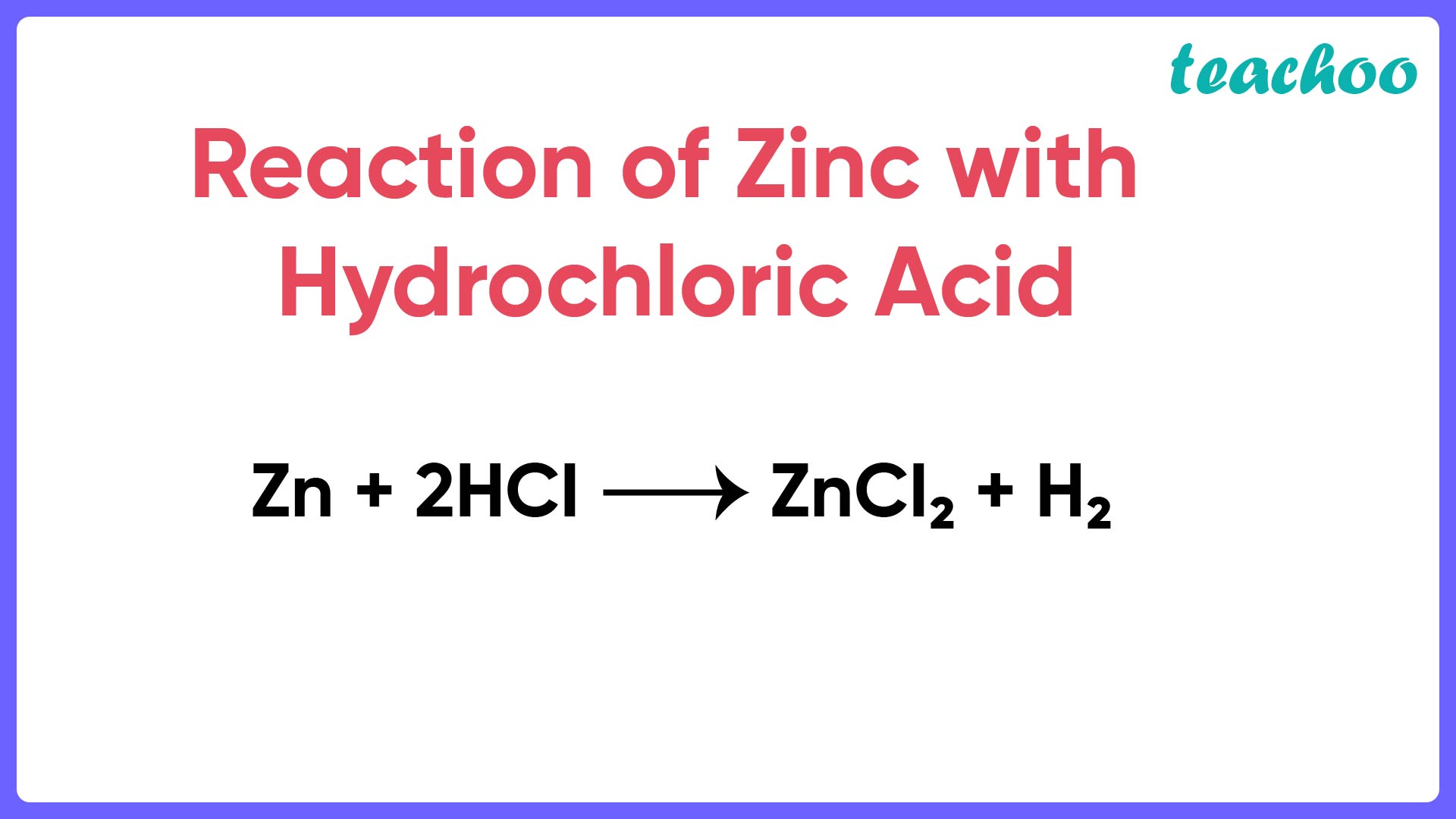
When Hydrochloric Acid And Zinc Are Combined . While energy changes are a potential sign of a chemical reaction, care Some other reactions absorb energy. Web learn how to predict the products and balance the equation of the single replacement. Web when zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. Acids will react with reactive metals, such as magnesium and. The (s) and (g) stand for solid and gas. Web a piece of metallic zinc (0.17g) reacts with a diluted water solution (1.0 ml, 5 wt. Web learn how zinc metal reacts with hydrochloric acid to form zinc chloride and. Web zinc metal reacts with hydrochloric acid according to the balanced equation: Learn about the properties and interactions. Web zinc reacts with hydrochloric acid to form zinc chloride and hydrogen gas. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. Zn(s) + 2h cl(aq) → zncl2(aq) +h 2(g) ↑⏐. The (aq) stands for the.
from www.teachoo.com
The (aq) stands for the. While energy changes are a potential sign of a chemical reaction, care Web learn how zinc metal reacts with hydrochloric acid to form zinc chloride and. Web a piece of metallic zinc (0.17g) reacts with a diluted water solution (1.0 ml, 5 wt. Web when zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. Web zinc metal reacts with hydrochloric acid according to the balanced equation: Learn about the properties and interactions. Web learn how to predict the products and balance the equation of the single replacement. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. Some other reactions absorb energy.
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro
When Hydrochloric Acid And Zinc Are Combined Acids will react with reactive metals, such as magnesium and. Acids will react with reactive metals, such as magnesium and. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. Web learn how to predict the products and balance the equation of the single replacement. Web zinc reacts with hydrochloric acid to form zinc chloride and hydrogen gas. Learn about the properties and interactions. Web zinc metal reacts with hydrochloric acid according to the balanced equation: While energy changes are a potential sign of a chemical reaction, care Web when zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. Zn(s) + 2h cl(aq) → zncl2(aq) +h 2(g) ↑⏐. Web a piece of metallic zinc (0.17g) reacts with a diluted water solution (1.0 ml, 5 wt. Some other reactions absorb energy. The (s) and (g) stand for solid and gas. The (aq) stands for the. Web learn how zinc metal reacts with hydrochloric acid to form zinc chloride and.
From www.alamy.com
Reaction of zinc with hydrochloric acid Stock Photo Alamy When Hydrochloric Acid And Zinc Are Combined Web a piece of metallic zinc (0.17g) reacts with a diluted water solution (1.0 ml, 5 wt. Web when zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. Some other reactions absorb energy. Acids will react with reactive metals, such as magnesium and. While energy changes are a potential sign of. When Hydrochloric Acid And Zinc Are Combined.
From readingandwritingprojectcom.web.fc2.com
zinc metal and hydrochloric acid When Hydrochloric Acid And Zinc Are Combined While energy changes are a potential sign of a chemical reaction, care Web zinc metal reacts with hydrochloric acid according to the balanced equation: Web a piece of metallic zinc (0.17g) reacts with a diluted water solution (1.0 ml, 5 wt. The (s) and (g) stand for solid and gas. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 (. When Hydrochloric Acid And Zinc Are Combined.
From www.youtube.com
Zinc and 6 M Hydrochloric Acid Reaction YouTube When Hydrochloric Acid And Zinc Are Combined Web a piece of metallic zinc (0.17g) reacts with a diluted water solution (1.0 ml, 5 wt. Web zinc metal reacts with hydrochloric acid according to the balanced equation: Web when zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. The (aq) stands for the. While energy changes are a potential. When Hydrochloric Acid And Zinc Are Combined.
From askfilo.com
13. Zinc and hydrochloric acid react according to the reaction Zn(s)+2HCl.. When Hydrochloric Acid And Zinc Are Combined The (s) and (g) stand for solid and gas. Web when zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. Learn about the properties and interactions. Acids will react. When Hydrochloric Acid And Zinc Are Combined.
From thewonderofscience.com
Reaction of zinc and hydrochloric acid (NY) — The Wonder of Science When Hydrochloric Acid And Zinc Are Combined Acids will react with reactive metals, such as magnesium and. The (aq) stands for the. Zn(s) + 2h cl(aq) → zncl2(aq) +h 2(g) ↑⏐. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. Web learn how zinc metal reacts with hydrochloric acid to form zinc chloride and.. When Hydrochloric Acid And Zinc Are Combined.
From www.youtube.com
Zinc and Hydrochloric Acid YouTube When Hydrochloric Acid And Zinc Are Combined Web learn how to predict the products and balance the equation of the single replacement. While energy changes are a potential sign of a chemical reaction, care Learn about the properties and interactions. Web zinc metal reacts with hydrochloric acid according to the balanced equation: Acids will react with reactive metals, such as magnesium and. Web learn how zinc metal. When Hydrochloric Acid And Zinc Are Combined.
From express.adobe.com
Zinc and Hydrochloric Acid When Hydrochloric Acid And Zinc Are Combined Web a piece of metallic zinc (0.17g) reacts with a diluted water solution (1.0 ml, 5 wt. The (aq) stands for the. The (s) and (g) stand for solid and gas. Web zinc reacts with hydrochloric acid to form zinc chloride and hydrogen gas. Learn about the properties and interactions. While energy changes are a potential sign of a chemical. When Hydrochloric Acid And Zinc Are Combined.
From slideplayer.com
Types of Chemical Reactions Ch ppt download When Hydrochloric Acid And Zinc Are Combined Acids will react with reactive metals, such as magnesium and. Web when zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. The (s) and (g) stand for solid and gas. Web zinc reacts with hydrochloric acid to form zinc chloride and hydrogen gas. Web a piece of metallic zinc (0.17g) reacts. When Hydrochloric Acid And Zinc Are Combined.
From express.adobe.com
Zinc and Hydrochloric Acid When Hydrochloric Acid And Zinc Are Combined Web learn how to predict the products and balance the equation of the single replacement. Some other reactions absorb energy. Web when zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. Zn(s) + 2h cl(aq) → zncl2(aq) +h 2(g) ↑⏐. Web zinc metal reacts with hydrochloric acid according to the balanced. When Hydrochloric Acid And Zinc Are Combined.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental When Hydrochloric Acid And Zinc Are Combined Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. Some other reactions absorb energy. Web when zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. Web learn how zinc metal reacts with hydrochloric acid to form zinc chloride. When Hydrochloric Acid And Zinc Are Combined.
From www.sciencephoto.com
Zinc reacts with hydrochloric acid Stock Image C052/7641 Science When Hydrochloric Acid And Zinc Are Combined Learn about the properties and interactions. Web a piece of metallic zinc (0.17g) reacts with a diluted water solution (1.0 ml, 5 wt. The (aq) stands for the. Web zinc reacts with hydrochloric acid to form zinc chloride and hydrogen gas. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined. When Hydrochloric Acid And Zinc Are Combined.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0309 Science When Hydrochloric Acid And Zinc Are Combined Web learn how to predict the products and balance the equation of the single replacement. The (s) and (g) stand for solid and gas. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. Web learn how zinc metal reacts with hydrochloric acid to form zinc chloride and.. When Hydrochloric Acid And Zinc Are Combined.
From readingandwritingprojectcom.web.fc2.com
zinc metal and hydrochloric acid When Hydrochloric Acid And Zinc Are Combined Web learn how to predict the products and balance the equation of the single replacement. Web zinc metal reacts with hydrochloric acid according to the balanced equation: While energy changes are a potential sign of a chemical reaction, care The (s) and (g) stand for solid and gas. Web zinc reacts with hydrochloric acid to form zinc chloride and hydrogen. When Hydrochloric Acid And Zinc Are Combined.
From fity.club
Zinc Reacts With Hydrochloric Acid When Hydrochloric Acid And Zinc Are Combined Web zinc reacts with hydrochloric acid to form zinc chloride and hydrogen gas. The (s) and (g) stand for solid and gas. Web when zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. The (aq) stands for the. Web learn how zinc metal reacts with hydrochloric acid to form zinc chloride. When Hydrochloric Acid And Zinc Are Combined.
From www.chegg.com
Solved When hydrochloric acid and zinc were combined, the When Hydrochloric Acid And Zinc Are Combined Web learn how to predict the products and balance the equation of the single replacement. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. Web zinc metal reacts with hydrochloric acid according to the balanced equation: Web a piece of metallic zinc (0.17g) reacts with a diluted. When Hydrochloric Acid And Zinc Are Combined.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental When Hydrochloric Acid And Zinc Are Combined Web learn how to predict the products and balance the equation of the single replacement. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. Web learn how zinc metal reacts with hydrochloric acid to form zinc chloride and. Some other reactions absorb energy. Web zinc reacts with. When Hydrochloric Acid And Zinc Are Combined.
From www.youtube.com
Reaction of Zinc and Hydrochloric acid YouTube When Hydrochloric Acid And Zinc Are Combined Web a piece of metallic zinc (0.17g) reacts with a diluted water solution (1.0 ml, 5 wt. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. Web learn how zinc metal reacts with hydrochloric acid to form zinc chloride and. The (aq) stands for the. Web zinc. When Hydrochloric Acid And Zinc Are Combined.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0622 When Hydrochloric Acid And Zinc Are Combined Some other reactions absorb energy. Web a piece of metallic zinc (0.17g) reacts with a diluted water solution (1.0 ml, 5 wt. Web learn how zinc metal reacts with hydrochloric acid to form zinc chloride and. Web learn how to predict the products and balance the equation of the single replacement. Web when zinc reacts with hydrochloric acid, the test. When Hydrochloric Acid And Zinc Are Combined.